| |
|
|
|
|
|
 |
| |
One of the chief usage of silicone products
is adhesive sealing.
There are various theories about the adhesion,
but we can take that the important factors
when trying to join different materials are
cleanliness of the surface, the appearance
of the surface, and the surface energy. Even
though they may be the surfaces of the metal
where the adhesive power of silicone is well
shown, we can hardly expect its own adhesive
power of silicone if the surface is stained
with some oil or so such as the cutting oil.
Moreover, from a more minute respective, a
rugged one tends to exert more adhesive power
than a slippery one because of its larger
contact area. |
|
Material |
Surface
energy (dyne/cm) |
|
Teflon |
18 |
|
Silicone |
20
~ 23 |
|
Polypropylene |
29
~ 30 |
|
polyethylene |
31
~ 32 |
|
Polyester |
35
~ 38 |
|
Various
metals |
Tens
~ Hundreds |
|
|
Surface enery is also
an important notion in the adhesion
theory, and the surface energies of
some materials are shown is the left
table Teflon can be regarded as the
material with the poorest adhesive power
because it has lower surface energy
than silicone, but various metals or
glasses can be regarded as the materials
that give good adhesive power. In other
words, materials with low surface energies
tend not to react and the those with
high surface energies tend to have high
reactive power. |
|
| There are many ways of inducing adhesion
in materials with poor adhesive power exertion,
and they sometimes do the sealing after spreading
the Primer, or they alter the surface to make
a good condition for adhesion exertion, or
sometimes they improve the adhesive power
by adjusting the type and amount of the silane
coupling agent used in silicone. |
| |
| Adhesion mechanism |
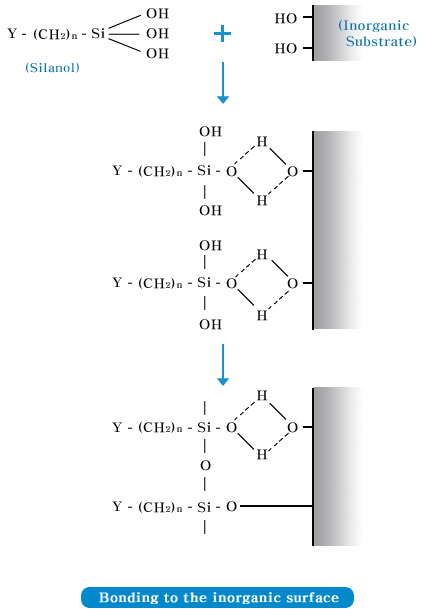 |
|
| The reaction mechanism of silane coupling
agent( Y - (CH2)n
- Si OR) used as the supplement of silicone
is as shown in the figure below. Si-OR of
silane coupling agent is hydrolyzed by water,
etc., forming Silanol, and this Silanol and
an inorganic surface forms a Si - O - M combination.
Also, other reactant Y is combined with an
organic material, and as a result an inorganic
material and an organic material are chemically
combined. |
| |
|